How to Decide Which Lewis Structure Is Best
The best lewis structure is B Lets have a look at the rules how we set up lewis structures most atoms want to have 8 electrons or 2 in the outer shell the valence shell. We recognize this kind of Ocs Lewis Structure graphic could possibly be the most trending subject behind we ration it in google plus or facebook.
Determine the total number of valence electrons in a molecule 2.
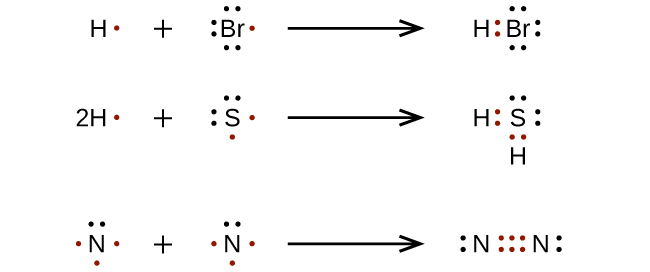
. While drawing the lewis dot structure for any chemical formula one needs to keep the following points in mind to avoid any mistakes. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. Calculating the formal charge is also a good tool.
Atoms in general dont like charges so having no charge is better. Choose the best Lewis structure for SO42-. You may need a periodic table for this.
Ocs Lewis Structure. O 5 O 4 O-1 O 2 Draw the Lewis structure for SO 2. The unbonded side of every atom then has a pair of dots.
Decide which is the central atom in the structure. The lewis structure with formal charges closest to 0 is best in the sense that makes a bigger contribution to the resonance hybrid than the other two resonance structures as is stated on page 73 of the course reader. Lewis structure A has a C atom singly bound to a O atom and a N atom.
This is called the octet rule Count all the valence electrons from the atoms involved in your structure. Once you have drawn a good Lewis structure your instructor will build the model. In this step add up the total number of valence electrons from all.
Following the rules for drawing lewis structures should help guide you in determining this. Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered. Use two valence electrons to form each bond inthe skeleton structure.
First determine the total number of valence electrons in the molecule. Draw the Lewis Dot Structure for the Hydrogen atom. To draw a Lewis structure the number of valence electrons on each atom in the compound must be determined.
When answering questions that involve resonance structures it is important to take into account all of the resonance structures and not only the. Determine the total number of valenceelectrons. Its essential for predicting molecular geometry molecule polarity and reactivity in a compound.
Yes when you are drawing lewis structures you should be evaluating your structure to determine if it is the most stable version. An atom is considered happy when its outer. The total number of valence electrons in the entire compound is equal to the sum of the valence electrons of each atom in the compound.
Find the Total Number of Valence Electrons. How to draw Lewis Diagrams. The Lewis structure of OCl2 has each Cl atom single bonded to an O atom in the center.
Its submitted by supervision in the best field. Lorain County Community College General Chemistry I CHMY 171 Using formal charge to determine the best Lewis structure. Steps for drawing Lewis Dot Structure.
D Draw the Lewis structure for the free radical NO2 and determine the formal charge on the N. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. O 30-4-03 ola ö.
How to Draw a Lewis Structure Step 1. Find the total number of valence electrons of hydrogen sulfur and oxygen atoms. 017 H 41 ö.
Choose the best Lewis structure for OCl2. Non-valence electrons are not represented when drawing the Lewis structures. To my knowledge there is not a way to know how many reasonable resonance structures.
That will normally be the least electronegative atom C. Determine the Number. Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
Try to satisfy the octets of the atoms bydistributing the remaining valence electrons as nonbondingelectrons. Here are the steps that I follow when drawing a Lewis structure. Determine the best Lewis structure of the following molecules.
Name the geometry and associated bond angle observed around each central atom. There are three electron pairs on O and three electron pai on N. How many equivalent resonance.
Since Hydrogen is in Group I it has one 1 valence electron in its shell. Selection for center atom. It will help to work out the missing formal charges for the atoms in these different structures Image description.
What is the formal charge on the central Cl atom. Draw the Lewis Structure. Draw a skeleton for the molecule which connects all atoms using only single bonds.
So Al is the central atom which has 3 valence electrons and there are no unbonded electrons as one of each is bonded to each Br however the valence shell of the central atom is not full therefore this. Search the total number of electron pairs. With these two questions we can determine whether this molecule is a lewis base or acid or you can always draw a Lewis Dot structure.
Choose the best Lewis structure for OCN. It is non-flammable in nature and bears a suffocating odor. Divide this number by 2 to obtain the number of electron pairs.
This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Here are a number of highest rated Ocs Lewis Structure pictures on internet. Choose the best Lewis structure for OCl2.
Begin drawing the Lewis dot structure of the molecule. Lewis structure B has a C atom singly bound to a O. Note that some of the molecules may have more than one central atom.
As described on page 73 of the course reading the lewis structure with formal charges closest to 0 is best in the sense that it makes a greater contribution to the resonance mixture than the other two resonance structures. We identified it from reliable source. This will be the sum of the group number a of all atoms plus the charge.
Write the skeleton structure of the molecule. Find the Number of Electrons Needed to Make the Atoms Happy. An outline of how to detemine the best Lewis structure for an example NO 3-is given below.
The following is an example of how to draw the best Lewis structure for NO 3- learning by example. In simple molecules the atom with the most available sites for bondng is usually placed central. The resonance structure with a complete octet is.

The Lewis Structure For Nco Chemistry Stack Exchange

How To Draw Lewis Structures Youtube

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube
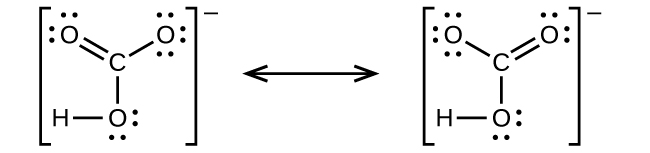
7 4 Formal Charges And Resonance Chemistry

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Exp Chemistry Classroom Teaching Chemistry Chemistry Lessons

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube

Bro3 Lewis Structure Bromate Ion How To Find Out Lewis Electrons
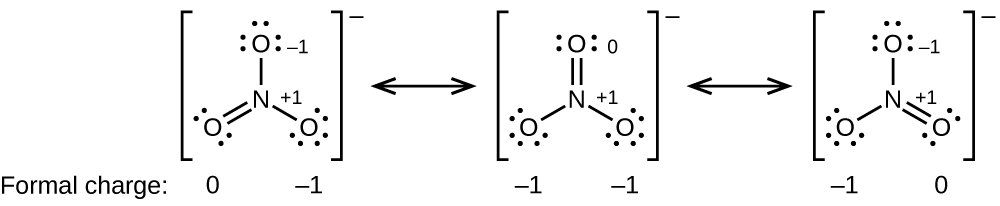
7 4 Formal Charges And Resonance Chemistry
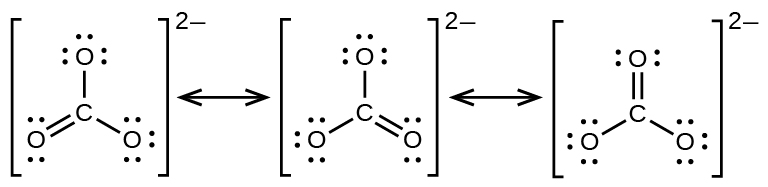
7 4 Formal Charges And Resonance Chemistry

Ncl3 Lewis Structure Nitrogen Trichloride How To Find Out Molecules Lewis

4 4 Drawing Lewis Structures Chemistry Libretexts
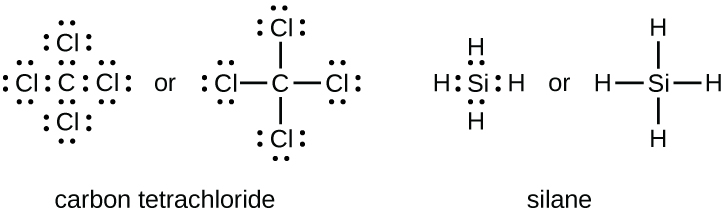
7 3 Lewis Symbols And Structures Chemistry
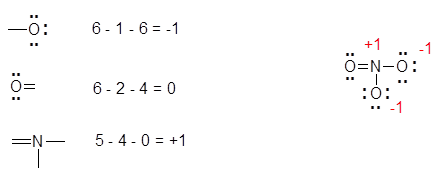
Lewis Structures Chemistry Libretexts
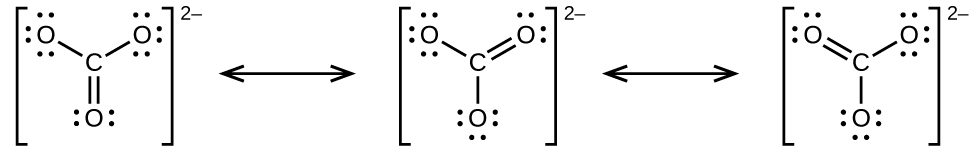
7 4 Formal Charges And Resonance Chemistry

Lewis Structures For Covalent Molecules Step By Step Youtube
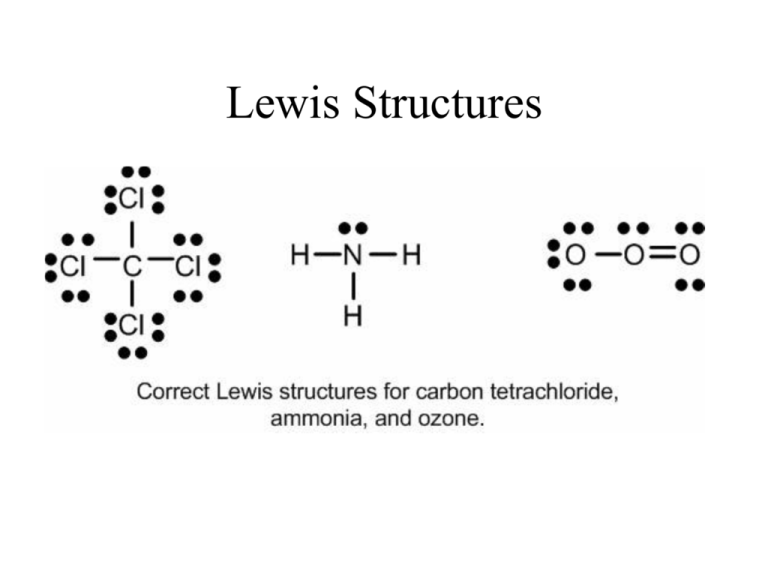
Comments
Post a Comment